Prepare the intestinal environment

Regulating the intestinal environment can help improve sleep. This is because gut bacteria have an influence on the stabilization of the host’s (person’s) circadian rhythm.
We cite and refer to the research paper 睡眠と腸内細菌叢.
The effect of sleep on intestinal function has long been known. This is evidenced by the high frequency of gastrointestinal symptoms among night workers and travelers. The mechanism has been postulated to be that sleep disturbance causes stress, which in turn leads to sympathetic activation and increased cortisol secretion, but recent molecular biological studies of intestinal bacteria have revealed diurnal variation in intestinal bacteria. It has also become clear that changes in sleep also affect the composition and function of intestinal bacteria. Furthermore, with the advancement of functional analysis of intestinal bacteria, it has become clear that changes in the function of intestinal bacteria brought about by changes in sleep can affect the energy metabolism of the host (human), triggering obesity and insulin resistance. The intestinal tract, a peripheral organ, is also influenced by clock genes, and circadian rhythms are observed in the type, proportion, and function of intestinal bacteria.
Sleep disturbances cause loss of circadian rhythm and alter composition of gut bacteria
Modulations such as jet lag and shortened sleep can alter the composition and function of intestinal bacteria and circadian rhythms.
The following article cites and references the research article Circadian Disorganization Alters Intestinal Microbiota.
明期と暗期を1週間ごとに変更する睡眠障害(通常は半日で明期と暗記が来ます)をマウスに与えたところ、大腸での概日リズムが消失しました。
In addition, mice on the high-fat, high-sugar diet showed an increase in Firmicutes and a decrease in Bacteroidetes in the intestinal microbiota compared to mice on the normal diet. The figure below illustrates this. In the figure below, NS (non-shifted) mice were grown under normal light and dark conditions, while S (shifted) mice were grown for one week under reversed light and dark conditions. In the comparison of NS and S mice, the gut microbiota did not change significantly. However, we can see that the change in diet has caused a significant change in the intestinal microflora. Firmicutes in mice on a normal diet were about 50%, but changed to more than 70% when the diet was changed to a high-fat diet. Bacteroidetes in mice fed a normal diet was about 40%, but in mice fed a high-fat, high-carbohydrate diet, it decreased to about 10%.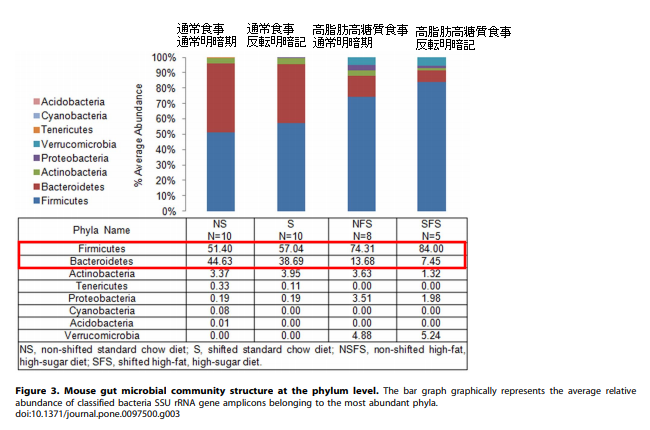
In another study, an intervention experiment was conducted to produce sleep disturbance by tactile stimulation for four weeks without changing the cycle of light and dark periods. The results showed an increase in Lachrospiraceae and Ruminococcaceae (at the family level of classification) and a decrease in Lactobacillaceae and Bifidobacteriaceae (at the family level of classification). These changes in intestinal bacteria disappeared 2 weeks after discontinuation of the sleep disorder.
Phenomena caused by changes in intestinal bacteria
Thus, changes in the composition of intestinal bacteria can cause a variety of phenomena.
The following article cites and references the research article Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice.
For example, metabolites in the intestinal tract are altered (decreased utilization of D-mannose, citric acid, and propionic acid). The intestinal contents of sleep-disordered mice decreased the barrier function of intestinal cell lines in vitro, suggesting that the intestinal bacteria of sleep-disordered mice may cause intestinal barrier dysfunction. In fact, sterile mice transplanted with intestinal bacteria from these sleep-deprived mice showed increased blood levels of proinflammatory cytokines such as IL-6 and exacerbated insulin resistance. Blood levels of Lipopolysaccharide-binding protein (LBP), which transports LPS to LPS receptors on the cell membranes of macrophages and other cells, were also increased in the sleep-disordered mice. This suggests that changes in the intestinal microbiota and impaired intestinal barrier function in the sleep-disordered model result in increased LPS influx into the blood.
The following article cites and references the research article Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals Christian.
When subjects are jet-lagged after two days of short sleep or eight hours of air travel, their intestinal bacteria increase in Firmicutes and decrease in Bacteroidetes. When the intestinal bacteria of the jet-lagged subjects were transplanted into sterile mice, accelerated body fat accumulation and worsened glucose tolerance (the ability to lower insulin resistance, the sensitivity to insulin, and to lower blood glucose to normal levels when blood glucose levels become high) were observed compared to mice that had been transplanted with normal intestinal bacteria. The bias had resolved in the intestinal bacteria of the subjects whose jet lag had resolved after 2 weeks of flight.
The following article cites and references the research article Oxalic acid and diacylglycerol 36:3 are cross-species
markers of sleep debt.
Other reported changes in blood metabolites when 10 hours of sleep is restricted to 4 hours have shown an increase in pipecolic acid, a metabolite of intestinal bacteria. Pipecolinic acid has been proposed as a diagnostic indicator of pyridoxine-dependent epilepsy.
The following article cites and references the research paper Intermittent hypoxia alters gut microbiota,
diversity in a mouse model of sleep apnoea.
The following article cites and references the research article Lipopolysaccharide-Binding Protein Plasma Levels in Children: Effects of Obstructive Sleep Apnea and Obesity.
Sleep disturbances may be accompanied by hypoxia, but hypoxia has also been reported to affect intestinal function and gut microbiota. Intermittent hypoxia has been shown to alter gut microbiota in mouse models, and in humans, sleep apnea has been reported to be associated with increased blood LBP independently of obesity. Intermittent hypoxia is thought to impair gut microbiota and intestinal barrier function.
Changes in the intestinal microflora can cause dysmetabolism of energy in the host (person), leading to the development of lifestyle-related diseases such as obesity and hypertension.
Circadian Rhythm and Sleep Regulation Possibly Mediated by Intestinal Bacteria
The following article cites and references the research article titled Effects of Diurnal Variation of Gut Microbes and High-Fat Feeding on Host Circadian Clock Function and Metabolism.
It has been shown using sterile mice that intestinal bacteria themselves can influence the circadian rhythm of the host. By comparing the expression of the clock genes Bmall and Clock in the medial basal hypothalamus and liver of sterile and normal mice, they found that circadian rhythms were observed in normal mice, but were attenuated in sterile mice. This phenomenon was also found to be unaffected by diet, indicating that the presence of intestinal bacteria is essential for the oscillation of regular circadian rhythms. In addition, intestinal bacteria produce a number of metabolites, among which is butyric acid. This butyric acid is instrumental in the formation of circadian rhythms in the intestinal tract. However, the fact that circadian rhythms were observed even after eating suggests that butyric acid and feeding are related to the formation of circadian rhythms.
Without the presence of intestinal bacteria, there is no butyrate production from the intestinal bacteria, so the circadian rhythm becomes unstable. Then sugar absorption increases and peptide absorption decreases. In addition, triglycerides and cholesterol increase.
Intestinal bacteria play a very important role in sleep, so make sure your intestinal environment is in good shape.


コメント